Synthesis and characterization of novel mesoporous and macroporous silicas
(1.1) Review on the research on anionic surfactant templated mesoporous silica
This tutorial review highlights the research on anionic surfactant templated mesoporous silica (AMS), which employs a co-structure directing agent (CSDA) to establish the critical interaction between the surfactant head group and silica species. As the geometry of anionic surfactants can be readily tuned via the ionisation of the surfactant head group, AMS materials possess a variety of mesostructures and morphologies. Chiral mesoporous silica (CMS) and helical ribbons can be formed via the chiral packing of the surfactant. Due to the pairing effect between the CSDA and the surfactant, a regular array of the organic groups is formed based on the stoichiometry and geometric arrangement of the surfactant, which produces functionalised materials with a uniform distribution of their organic groups. Furthermore, a brief introduction to the applications and future requirements of AMS is also included. This review is addressed to researchers and students interested in diverse areas of chemistry, particularly inorganic, physical, supramolecular and materials chemistry. This review has been published on Chem. Soc. Rev., 2013, 42, 3740.
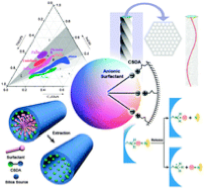
(1.2) Synthesis of mesoporous materials by Co-structure Directing Agent
Anionic surfactants are used in greater volume than any other surfactants because of their highly potent detergency and low cost of manufacture. However, they have not been used as templates for synthesizing mesoporous silica. Here we show a templating route for preparing mesoporous silicas based on self- assembly of anionic surfactants and inorganic precursors. We use aminosilane or quaternized aminosilane as co-structure-directing agent (CSDA), which is different from previous pathways. The alkoxysilane site of CSDA is co-condensed with inorganic precursors; the ammonium site of CSDA, attached to silicon atoms incorporated into the wall, electrostatically interacts with the anionic surfactants to produce well-ordered anionic-surfactant-templated mesoporous silicas (AMS). These have new structures with periodic modulations as well as two-dimensional hexagonal and lamellar phases. The periodic modulations may be caused by the coexistence of micelles that differint in size or curvature, possibly owing to local chirality. These mesoporous silicas provide a new family of mesoporous materials as well as shedding light on the structural behaviour of anionic surfactants. This work has been published on Nature Materials, 2003, 2, 801.
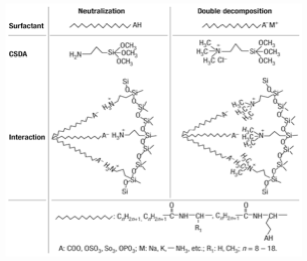
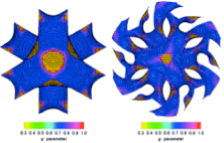
(1.3) Formation mechanism of the anionic surfactant templated mesoporous silicas
Cage−type structure , two−dimensional (2D) cylindrical hexagonal ( C ) , bicontinuous gyroid ( G ) , bicontinuous diamond ( D ) and lamellar ( L ) mesophases have been obtained by using amino acid derived anionic amphiphilic molecules N −stearoyl−l−glutamic acid (C 18 GluA) as the template , 3−aminopropyl−trimethoxysilane (APS) as CSDA and tetraethyl orthosilicate (TEOS) as the silica source , in the presence of nonionic co−surfactant C 16 (EO) 10 (Brij−56). The mesophases changed in an order that cage−type → C → intergrowth of C and D → intergrowth of C and G → D → G → L , with the increase of the Brij−56/C 18 GluA molar ratio. The mesophases tranformation was explained by the organic/inorganic interface curvature change caused by the hydrophobicity and penetration effect of Brij−56. The structural relationship with the surface curvature analysis of the mesophases were investigated , furthermore , the observed intergrowth structures have been studied in detail. Besides , by using electron crystallographic method , the actual g parameters of D and G obtained in this system were determined and compared. This work has been published on J. Am. Chem. Soc., 2011, 133, 11524.
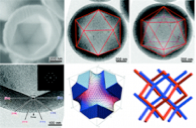
(1.4) Formation mechanism of the anionic surfactant templated mesoporous silica hollow sphere
A novel hollow sphere with remarkable polyhedral crystal−like hollow morphology and a highly ordered double diamond bicontinuous mesoporous shell has been formed in a unique crystal growth system. Vesicle with a low−curvature lamellar structure was first formed by the self−assembling of amino acid derived amphiphilic carboxylic acid in the presence of a non−ionic surfactant, which followed a structural transformation to a highly ordered crystalline mesoporous shell with double diamond structure by maintaining a hollow cavity. The formation of the reversed multiply twinned particle (MTP) led to the icosahedral/decahedral hollow shape, and the sphere achieved the lowest total free energy by maximizing the spherical surface coverage. This work has been published on J. Am. Chem. Soc., 2011, 133, 6106.